The Top 4 Reasons why Electronic Clinical Outcome Assessment (eCOA) clinical trials are considered far more complex than Electronic Patient-Reported Outcome (ePRO) studies due to several reasons.
- Data Collection: eCOA trials require more comprehensive and accurate data collection compared to ePRO studies. eCOA trials use electronic devices such as tablets or smartphones to gather data from patients, which is then transmitted to a central database. This data collection process is more complex than ePRO studies as it requires the integration of various software and hardware systems.
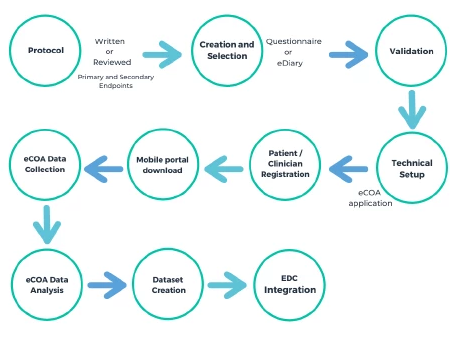
- Data Management: The data collected through eCOA trials is much more complex and varied compared to ePRO studies, which makes it challenging to manage. In eCOA trials, the collected data is typically more comprehensive, including multi-modal data such as videos, images, and audio recordings. This requires more sophisticated data management systems and processes to ensure that the data is accurate, complete, and secure.
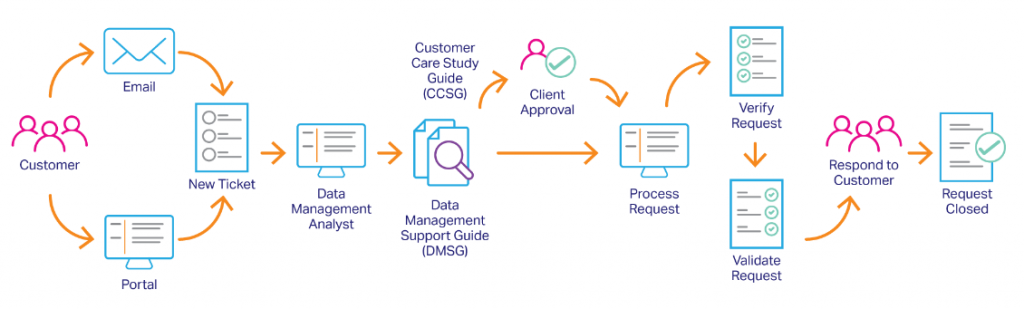
- Compliance and Regulation: eCOA trials are subject to strict regulatory requirements, such as the 21 CFR Part 11 and the EU General Data Protection Regulation (GDPR), which require robust and secure data management systems. These regulations also require that data be collected in a manner that ensures the privacy and confidentiality of patients. eCOA trials must also comply with various other regulations such as the FDA’s guidance on Electronic Source Data in Clinical Investigations.

- Study Design: eCOA trials often involve more complex study designs compared to ePRO studies, which can increase the complexity of the trial. For example, eCOA trials may involve multi-site, multi-country, and multi-lingual studies. This requires coordination and collaboration among multiple parties, including clinical sites, regulatory authorities, and data management teams, which can be challenging.
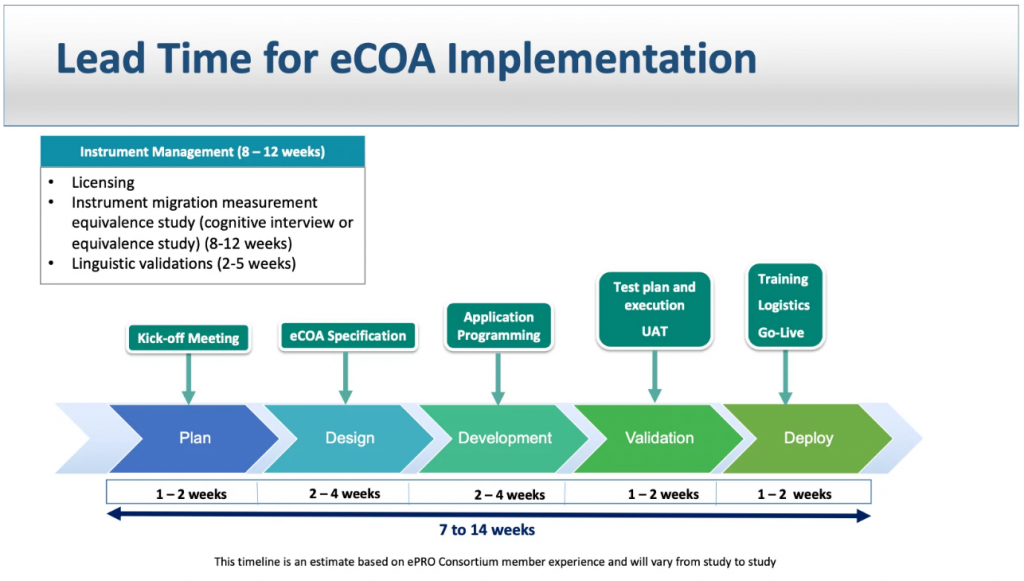
eCOA clinical trials are more complex than ePRO studies due to the more comprehensive and accurate data collection, the more complex data management, the need to comply with strict regulatory requirements, and the more complex study designs. Despite the challenges, eCOA trials offer many benefits over traditional methods, including increased patient engagement, improved data quality, and reduced time and cost.
